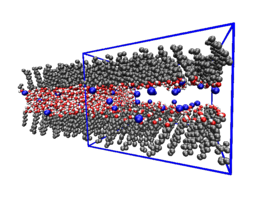
The interaction between charged surfaces in water is fundamental for physical chemistry and biology, since basically all surfaces become charged when immersed in water. Experimentally measured surface interactions can be well described by the additive contributions of electrostatic interactions (in the form of Poisson-Boltzmann theory), dispersion interactions and, at nanometer separations, hydration repulsion. However, a stringent test of theoretical predictions has so far been impossible, because the surface charge density and the location of the surface charge is experimentally never known precisely and thus enter the theory as fit parameters. Such a test should in principle be possible with water-explicit simulations of charged surfaces, since here the surface charge distribution is exactly known; but keeping the water chemical potential fixed (which is the experimentally realized ensemble) has so far been difficult in simulations.
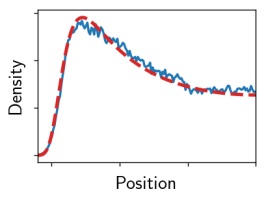
In the last years we have refined simulation techniques to control the water chemical potential [1]. In our recent paper (missing reference) we report simulations of charged surfaces in the presence of monovalent counterions and water at fixed chemical potential and compare the resulting surface interaction pressures with theoretical predictions. While our simulated counterion density profiles are accurately described by a modified Poisson-Boltzmann approach, the simulated interaction pressures are only for low surface charges described by the additive contributions of hydration and Poisson-Boltzmann repulsion. Already for moderately high surface charge densities this additivity assumption breaks down.
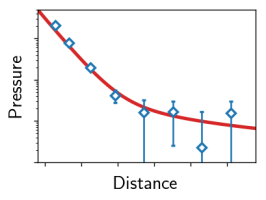
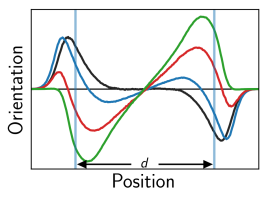
This we rationalize by a combination of different effects, namely, counterion correlations as well as the surface charge-induced reorientation of hydration water, which modifies the effective water dielectric constant [2]. Whereas the reduction of the water dielectric constant in confinement has recently been confirmed experimentally, a detailed analysis for charged and confined systems remains a challenging task and is nontrivial even in bulk electrolytes. Finally, the pronounced water reorientation is expected to strongly influence the hydration repulsion itself, revealing that the analysis of pressure-distance curves for charged surfaces in water at nanometer separation usually is subject to a large number of unknowns.
- A. Schlaich, B. Kowalik, M. Kanduč, E. Schneck, and R. R. Netz, “Simulation Techniques for Solvation-Induced Surface-Interactions at Prescribed Water Chemical Potential,” in Computational Trends in Solvation and Transport in Liquids, vol. 28, G. Sutmann, J. Grotendorst, G. Gompper, and D. Marx, Eds. Jülich: Forschungszentrum Jülich GmbH, 2015, pp. 155–185. [WEB]
- A. Schlaich, E. W. Knapp, and R. R. Netz, “Water Dielectric Effects in Planar Confinement,” Phys. Rev. Lett., vol. 117, no. 4, p. 048001, Jul. 2016. [WEB][DOI]